ISO 13485 Certification : Medical Devices - Quality Management System

What is ISO 13485 Certification?
ISO 13485 Certification is an international standard providing the framework for Qualtity management system for medical device industries. ISO 13485 Certification is the basis for maintaining a top-quality management system for medical device units. It should be noted that, if you are manufacturing any medical-based finished products then ISO 13485 Certificate is the ultimate requirement for your organization to assure the quality and efficiency of the products. So, it is very important for the manufacturers of medical devices to ensure the quality and safety of these devices to their customers as meet the related regulatory requirements for the product.
Why is ISO 13485 Certification :Medical Devices Quality Management System important for you?
Being an ISO 13485 certified professional imposes your determination to provide qualitative-products & services to your organization and clients. Attaining the necessary knowledge and skills to operate an ISO 13485 Certification framework demonstrates your commitment to supporting your organization ensure regularly improvement and best work-processes. ISO 13485 standard also indicates that you understand the importance of the safety & performance of medical-devices and how a medical devices quality management system (MD-QMS) can insure just that, consequently leading to client satisfaction. Similarly, ISO 13485 Certification can introduce you to new opportunities such as working for large organizations that provide best service and quality products. These organizations will value your knowledge & expertise on this standard, while enabling you to maximize your earning-potential.
ISO 13485 Certification Principles:
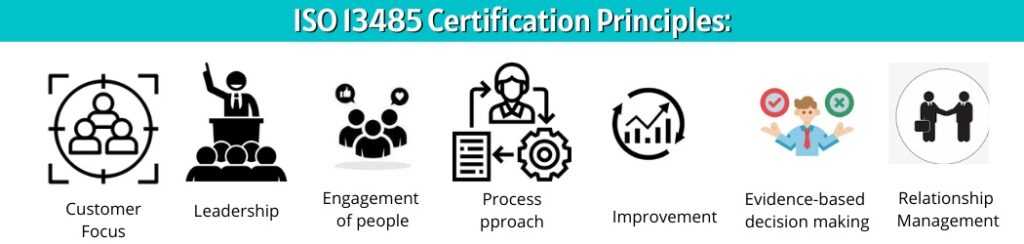
- Customer-focus
- Leadership
- Engagement of people
- Process-approach
- Improvement
- Evidence-based decision making
- Relationship management
Advantage of Obtaining ISO 13485 Certification:
While beneficial for any Medical device manufacturer, ISO 13485 implementation and certification is a very good starting point for organizations seeking to enter the medical-device-industry. In particular, certified companies can:
- Create a culture of continual-improvement;
- Prove ability to meet customer and legislative requirements.
- Demonstrate commitment to safety & quality
- Improve ability to contract with larger organizations;
- Improve internal processes and decision-making;
Encourage international recognition
ISO 13485 certification helps to have huge opportunities for your business operations in the global marketplace which helps you to get international recognition. ISO 13485 certification ensures the quality and safety of medical devices to your customers, making you a preferred choice while doing business. This will also help your organization to earn maximum profits.
Boosts your image in the global market
ISO 13485 boosts your reputation in the market for being committed to the quality and efficiency of medical devices. This helps you to grasp huge business opportunities that are beneficial for your manufacturing industry. It helps you in gaining a lot of business opportunities for your industry.
Ensures customer satisfaction
Nowadays, ISO 13485 certification has become an important requirement for almost every manufacturing industry that deals with medical devices. ISO 13485 guarantees customer satisfaction and meets their requirements which in turn helps to retain those customers for a longer period.
Gives you a competitive edge
ISO 13485 certification is becoming a primary requirement to do business. Many vendors and subcontractors are required to conform to the specifications of the ISO 13485 standard. Therefore, with this certification, you can become a key player in the supply chain of medical devices.
Improves the quality of your medical devices
The ISO 13485 certification provides the management system for Quality Management systems for medical devices which ensures that your processes are effective and efficient enough to deliver premium quality medical products consistently meeting the customers’ or clients’ expectations.
What is the purpose of ISO 13485 Certification?
Adopting ISO 13485 Certification offers a practical-foundation for manufacturers to address the EU Medical Device Directive (MDD), the EU-Medical Device Regulation (MDR), and other regulations, as well as demonstrating a commitment to the safety & quality of medical-device.
Starting with management-support & identifying the customer needs for the quality management system, you will require to start developing documentation including the Quality-Policy, Quality-Objectives, and Quality-Manual. Together, these define the overall scope and implementation of the Quality Management System of medical devices. Along with these, you will require to create the compulsory and additional processes and procedures necessary for your organization to properly create and deliver your services and product.
ISO 13485 Certifications Strengthens Medical Device manufacture organizations
The medical device business sector is a high pressure industry where people praise-innovation as long as it does not sacrifice customer safety. Moreover, Medical devices manufacturers must respond to demand-spikes, as instances like the surge in ventilator requirements during the COVID-19 pandemic situation.
Getting an ISO 13485 certification can support a organizations make positive, permanent quality and process improvements. Thus, representatives from certified organizations often find that ISO 13485 Certification contributes to organizational resilience. It is not good for every organization, but the associated advantage makes it well worth consideration.
What Are the Estimated Costs of ISO 13485 Certification?
This is a very-very difficult area to address. Determining whether this project will be conducted in-house or out-side is necessary. The amount of time allocated by staff/employee (and the different-different departments), as well as training costs, should be accounted for in the cost analysis.
The Requirements of ISO 13485 Certification:
The structure of ISO 13485 Certification consists of 10-segments, out of which the first three are introductory in nature, whereas the last seven specifies the requirements of Quality Management Systems for medical devices, against which ISO 13485 certification is performed.
The Requirements of ISO 13485 Certification:
Clause 4: Context of the organization – This section deals with understanding the uniqueness of the organization of your medical-devices and customizing ISO 13485 certification in order to implement a quality management system that is most suitable for your medical devices industry.
Clause 5: Leadership – This section highlights the importance of top management in the implementation of a quality management system for the medical device industry by proper evaluation of the risks, planning actions, and assigning responsibilities to the relevant staff.
Clause 6: Planning – This section analyses the risks and opportunities for a quality management system for your medical devices, the top management is always expected to plan and enlist the quality-based objectives for your medical devices industry.
Clause 7: Support – This section points out all the resources, such as human resources, infrastructure, and others that are required for an effective quality management system for your medical devices industries. This section demonstrates the requirements around competence, awareness, communication, and controlling documented information.
Clause 8: Operation – This section deals with the implementation part of the planning for the quality management system for the medical device industry. It includes product requirements assessment, keeping scrutiny on external providers, analysing the product before release, and so on.
Clause 9: Performance evaluation – This section deals with the methods by which you can ensure the effective functioning of your quality management system for your medical devices. It involves regular management inspection, observing and measurement of the techniques, etc.
Clause 10: Improvement – This section ensures that the quality management system of your medical devices is upgraded and is capable of meeting the current market requirements. It involves regular scrutiny to identify gaps as well as performing corrective actions to close those gaps for continual improvement.
Clause 5: Leadership – This section highlights the importance of top management in the implementation of a quality management system for the medical device industry by proper evaluation of the risks, planning actions, and assigning responsibilities to the relevant staff.
Who should be ISO 13485 certified?
The ISO 13485 certification is a assure of Quality Management System compliance to the standard for organizations involved in the Medical-Device-industry. This approach is not only followed by Medical-Device-Manufacturers but also other supporting-organizations such as Sub-contractors, Suppliers, European Authorized Representatives, specialized Consulting firms and other…
Major differences between ISO 13485 Certification and ISO 9001 Certification
| |
|
| stage of the medical devices |
|
| devices in conformance with customer and regulatory requirements |
|
| regulatory requirements |
or negative on an organization’s QMS. |
| Quality Manual and role of management representatives. |
|
Why should you choose QMCS(INDIA) FOR ISO 13485 Certification consultancy?
Cost- We are committed to provide high-quality work, catering to your specific requirements at a surprisingly affordable cost!
Speed- We feel the pain when a business waits too long for ISO 13485 certification. It is usually followed by feelings of uncertainty and tension. Compliance support will prepare your organization in a way that you can complete the ISO certification process and get the certification within 90 days or less depending upon the internal support and basic system in place.
Ample Resources- ISO 13485 certification is not only a lengthy process but also a complicated in that it can need a more resources. If a lack of in-house resources is your main concern, leave it to us. Compliance support has skilled and experienced ISO 13485 Certification consultants who can easily fulfil your ISO requirements.
Self Managed –At Compliance support, our ISO 13485 Certification consultant believe in the power of a lean approach to consulting. This means that our customer/clients are more able to retain their ISO certification without the requirement for ongoing consulting support.
History of ISO 13845 Certification
ISO 13485 Certification was published in 1996 as standard for medical device companies based on iso 9001.
How does QMCS (INDIA) Support your organization to get ISO 13485 Certification?
QMCS (INDIA), one of the leading ISO Consultancy in India, Those support your organization to get ISO 13485 certification for your medical devices industry by using all the right processes. QMCS (INDIA), give all the information about the procedure for acquiring ISO 13485 for quality management system of your medical devices. It provides guidance to your organization for getting ISO 13485 certified management system.
QMCS (INDIA), support the applicant to be well aware of the importance of ISO 13485 in their manufacturing unit. It support them to remain competitive in several business spheres, including all the efficient and effective processes for improvement in the operations. QMCS (INDIA), Focus at delivering effective, practical, and result-oriented solutions for your medical device industry.
Where We offer ISO 13485 Certification Consultancy Services :
ISO 13485 Certification in Faridabad
ISO 13485 Certification in Gwalior
ISO 13485 Certification in Shimla
ISO 13485 Certification in Solan
ISO 13485 Certification in New Delhi
ISO 13485 Certification in Kanpur
ISO 13485 Certification in Patna
ISO 13485 Certification in Manesar
ISO 13485 Certification in Dharuheda
ISO 13485 Certification in Okhala
ISO 13485 Certification in Neharoo Place
ISO 13485 Certification in Sohana
ISO 13485 Certification in Bhallabhgarh
ISO 13485 Certification in Surat
ISO 13485 Certification in Haridwar
ISO 13485 Certification in Mumbai
ISO 13485 Certification in Bhopal
ISO 13485 Certification in Pune
ISO 13485 Certification in Mumbai
ISO 13485 Certification in Hyderabad
ISO 13485 Certification in Chennai
ISO 13485 Certification in Jaipur
ISO 13485 Certification in Jodhpur
ISO 13485 Certification in Kota
ISO 13485 Certification in Bangalore
ISO 13485 Certification in Ajmer
ISO 13485 in Tiruvanantapuram
ISO 13485 Certification in Kolkata
ISO 13485 Certification in Jammu
ISO 13485 Certification in Ranchi
ISO 13485 Certification in Jaipur
ISO 13485 Certification in gangtok
ISO 13485 Certification in Shimla
ISO 13485 Certification in surat
ISO 13485 Certification in Gandhinagar
ISO 13485 Certification in Ahemdabad
ISO 13485 Certification in Amravati
ISO 13485 Certification in Dispur
ISO 13485 Certification in Patna
ISO 13485 Certification in Panaji
ISO 13485 Certification in Bangaluru
ISO 13485 Certification in Aizawl
ISO 13485 Certification in Bhubaneswar
ISO 13485 Certification in Agartala
ISO 13485 Certification in Deharadun
ISO 13485 Certification in Daman
ISO 13485 Certification in Pune
ISO 13485 Certification in Delhi
ISO 13485 Certification in Delhi
ISO 13485 Certification in Chandigarh
ISO 13485 Certification in Sonipat
ISO 13485 Certification in Panipat
ISO 13485 Certification in Amritsar
ISO 13485 Certification in Kanpur
ISO 13485 Certification in Lucknow
ISO 13485 Certification in Indor
ISO 13485 Certification in Noida
ISO 13485 Certification in Gurgoan
ISO 13485 Certification in Delhi NCR
ISO 13485 Certification in Ambala
ISO 13485 Certification in Agra
ISO 13485 Certification in Faridabad
ISO 13485 Certification in kanpur
